Nuclear fusion refers to the process by which multiple nuclei of similarly charged atoms unite to form a heavier atom and typically A LOT OF ENERGY--with the exception of atoms heavier than iron, which actually absorb energy on fusion. Due to the typically high energy output, nuclear fusion could potentially provide a useful source of energy for our growing--and increasingly demanding--global population. One significant problem is that the process typically occurs only at ridiculously high temperatures, such as those found in the Sun or in a hydrogen bomb. Not surprisingly, scientists have long been interested in designing cheap, safe and effective methods for controlled fusion to produce usable energy.
Pons and Fleischman described a system containing deuterated water (D2O), two palladium electrodes, and a current running through the electrodes. They claimed that the current caused the palladium electrodes to absorb deuterium atoms, which were then forced so closely together that they underwent nuclear fusion to produce neutrons and energy in the form of heat: "...fusion occurs, out of that comes one or two new elements of less mass, and the difference is the energy that comes out. And that then would boil water, essentially. And when you boil water, you can make steam. And when you make steam, you can drive a turbine. And if you can drive a turbine, you can create electricity..." (watch the press conference).
Skeptical scientists immediately tried to reproduce a similar cold fusion and they found largely inconsistent results. Many suggested that the heat Pons and Fleischman observed was produced either by the current or by reactions in the water.











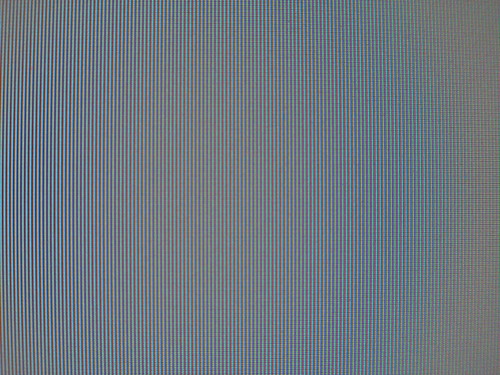




 where k is the reaction rate constant, A is a "pre-exponential factor," which depends on the order of the reaction, R is the universal gas constant, and T is temperature. It is clear that if the temperature increases, the rate constant will increase as well. When considering common reactions that occur at room temperature, people often say that the rate of the reaction roughly doubles for every 10 degree increase in temperature. Although this is a useful generalization to keep in mind, it is only true when the energy of activation is low (on the order of 10 kcal/mol). Otherwise, this statement does not hold.
where k is the reaction rate constant, A is a "pre-exponential factor," which depends on the order of the reaction, R is the universal gas constant, and T is temperature. It is clear that if the temperature increases, the rate constant will increase as well. When considering common reactions that occur at room temperature, people often say that the rate of the reaction roughly doubles for every 10 degree increase in temperature. Although this is a useful generalization to keep in mind, it is only true when the energy of activation is low (on the order of 10 kcal/mol). Otherwise, this statement does not hold.





